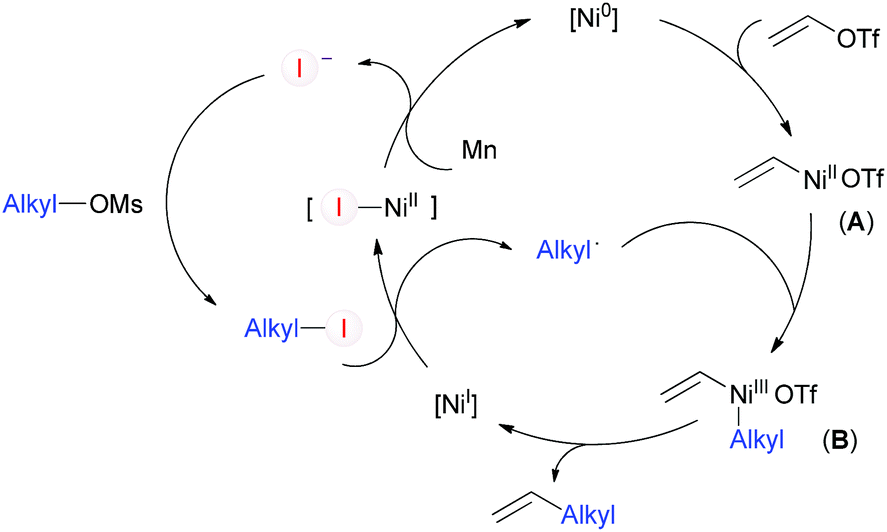
Alkyl Vinyl Aryl Acyl Halide Bonds

A vinyl halide is clearly a species with a formula h 2c c x h in which a halide is directly bound to an olefinic bond formally this is ethylene h 2c ch 2 with one of the hydrogens substituted by a heteroatom vinyl chloride h 2c chcl is an example.
Alkyl vinyl aryl acyl halide bonds. Are subdivided into alkyl vinylic aryl and acyl halides. The extra strength of the carbon halogen bond in aryl halides. For example if the halogen atom is attached to a carbon atom which is attached to a benzene ring cl ch 2 c 6 h 5 one would think it is an aryl halide but it is an alkyl halide because the halogen atom is attached to the carbon that is sp 3 hybridized. Other articles where vinylic halide is discussed.
In aryl halides the halogen bearing carbon is part of. In alkyl halides all four bonds to the carbon that bears the halogen are single bonds. Both alkyl and aryl groups have carbon and hydrogen atoms. In addition the carbon halogen bond is shorter and therefore stronger in aryl halides than in alkyl halides.
The main difference between alkyl and aryl groups is that alkyl groups do not have aromatic rings whereas aryl groups have aromatic rings in their structure. Alkanoyl is usually derived from a carboxylic acid therefore it has the formula rc o where r represents an alkyl group that is linked to the carbon. Halogen metal exchange of aryl halides. Halogens are more electronegative than carbon.
In aryl halides the halogen bearing carbon is part of an. An acyl group is a moiety derived by the removal of one or more hydroxyl groups from an oxoacid including inorganic acids it contains a double bonded oxygen atom and an alkyl group r c o. Alkyl halides have little to no solubility in water but be aware of densities. The halogen atom of an aryl halide atom could be exchanged for a metal atom using an organometallic reagent.
An example of such reaction is the reaction between bromobenzene and an organolithium reagent where there. They are subdivided into alkyl vinylic aryl and acyl halides. However alkyl halides may sometimes be confused with aryl halides. The attraction between the alkyl halide molecules is stronger than the attraction between the alkyl halide and water.
Organometallic compounds are compounds that have a bond between a carbon and a metal atom. Likewise phenyl cations are unstable thus making s n 1 reactions impossible. There is an interaction between one of the lone pairs on the chlorine atom and the delocalized ring electrons and this strengthens the bond. Steric hindrance caused by the benzene ring of the aryl halide prevents s n 2 reactions.
In organic chemistry the acyl group iupac name. Other articles where aryl halide is discussed. In vinylic halides the carbon that bears the halogen is doubly bonded to another carbon. In alkyl halides all four bonds to the carbon that bears the halogen are single bonds.
Alkyl groups and aryl groups are two examples of functional groups. An aryl halide has general formula c 6h 5x in which an halide group x has substituted the aryl ring. In vinylic halides the carbon that bears the halogen is doubly bonded to another carbon.