Allylic Vs Vinylic Carbocation

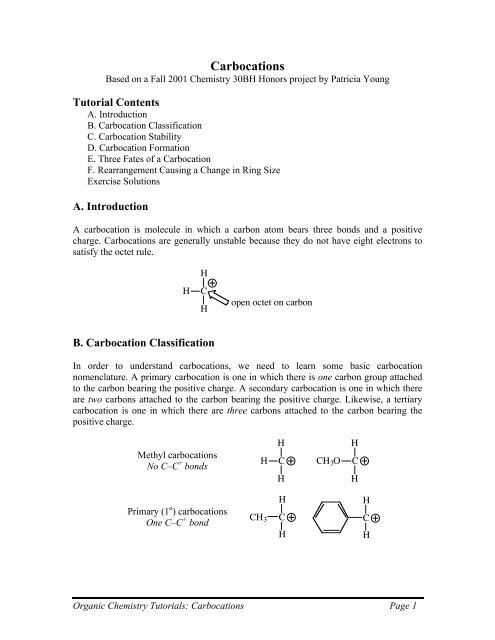
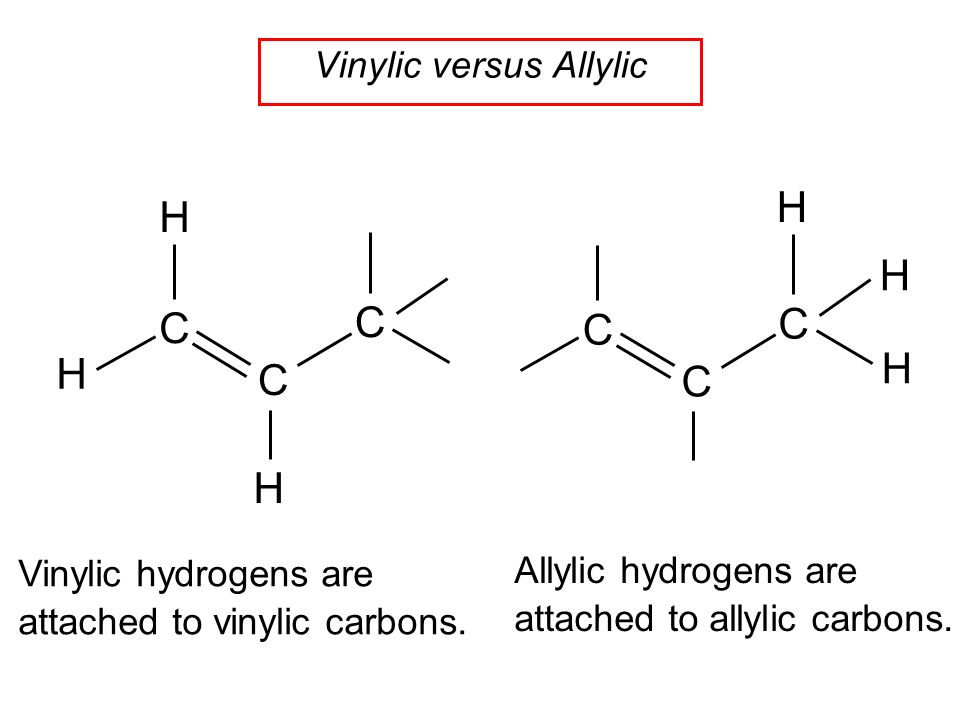
Atoms or groups attached to an allylic carbon are termed allylic substituents.
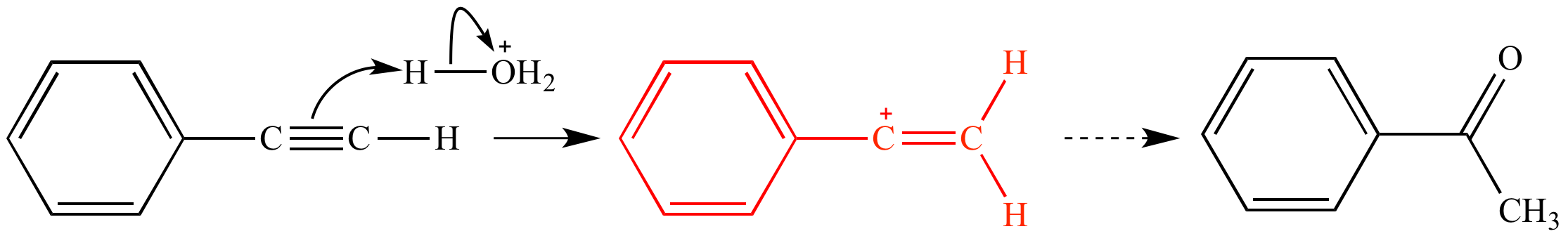
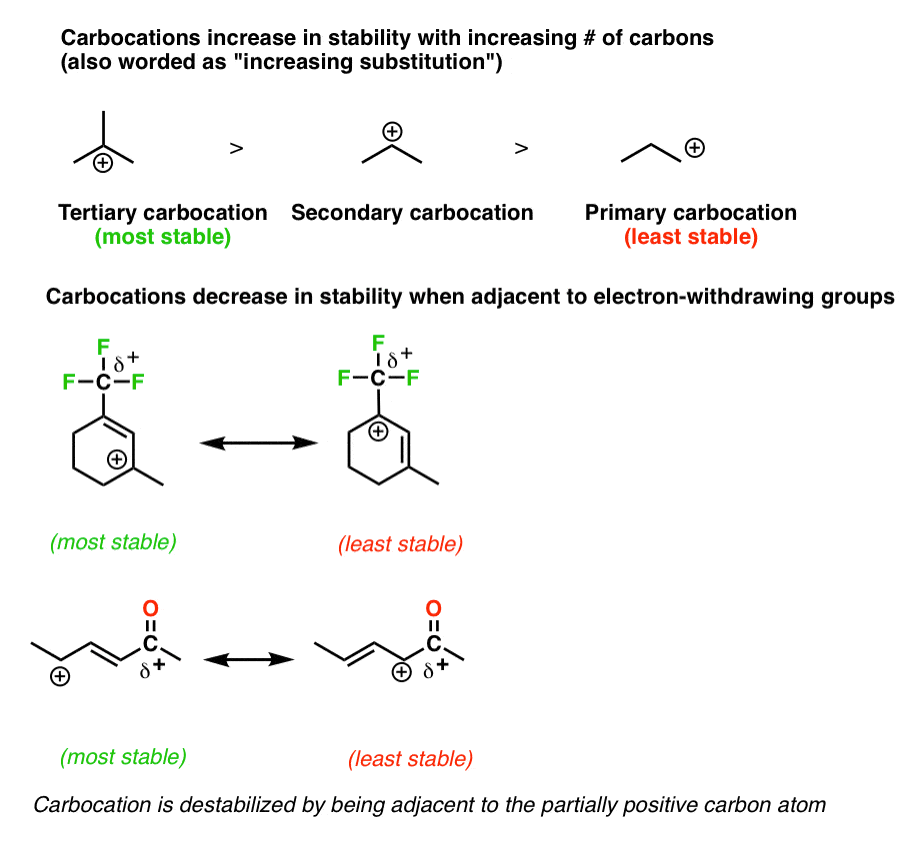
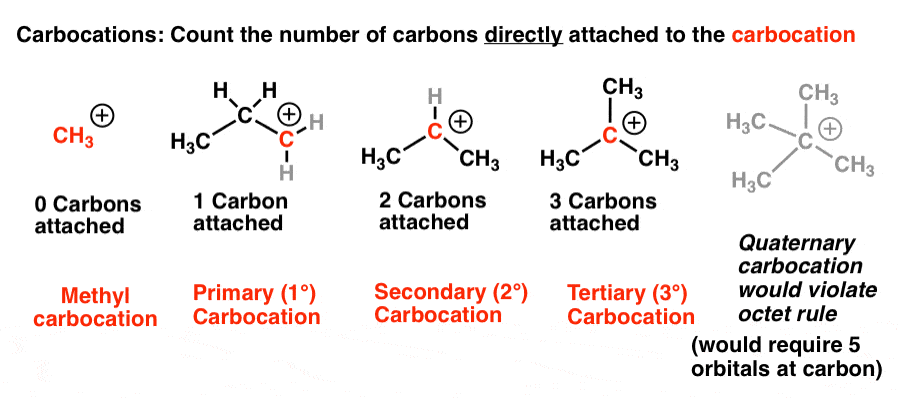
Allylic vs vinylic carbocation. Vinylic carbon is a carbon that is involved in a double bond with another carbon. In the tertiary carbocation shown above the three alkyl groups help to stabilize the positive charge. What is vinylic carbon. Compounds related to the vinyl cation include allylic carbocations and benzylic carbocations as well as aryl carbocations.
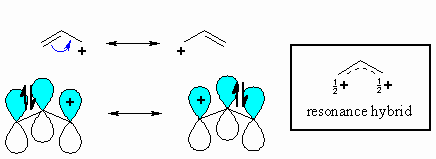
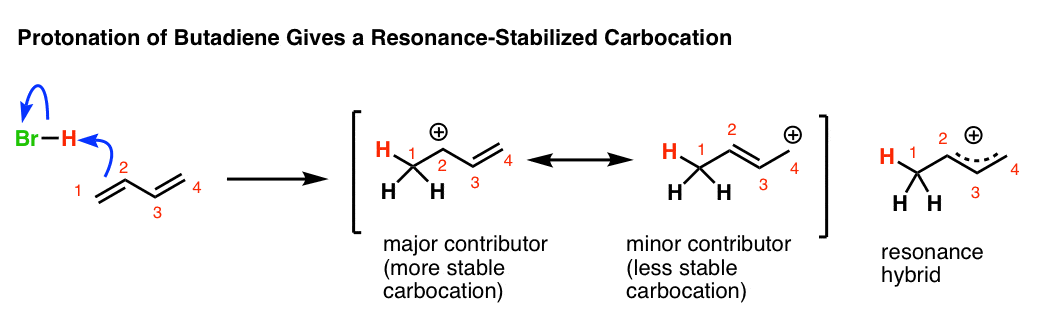
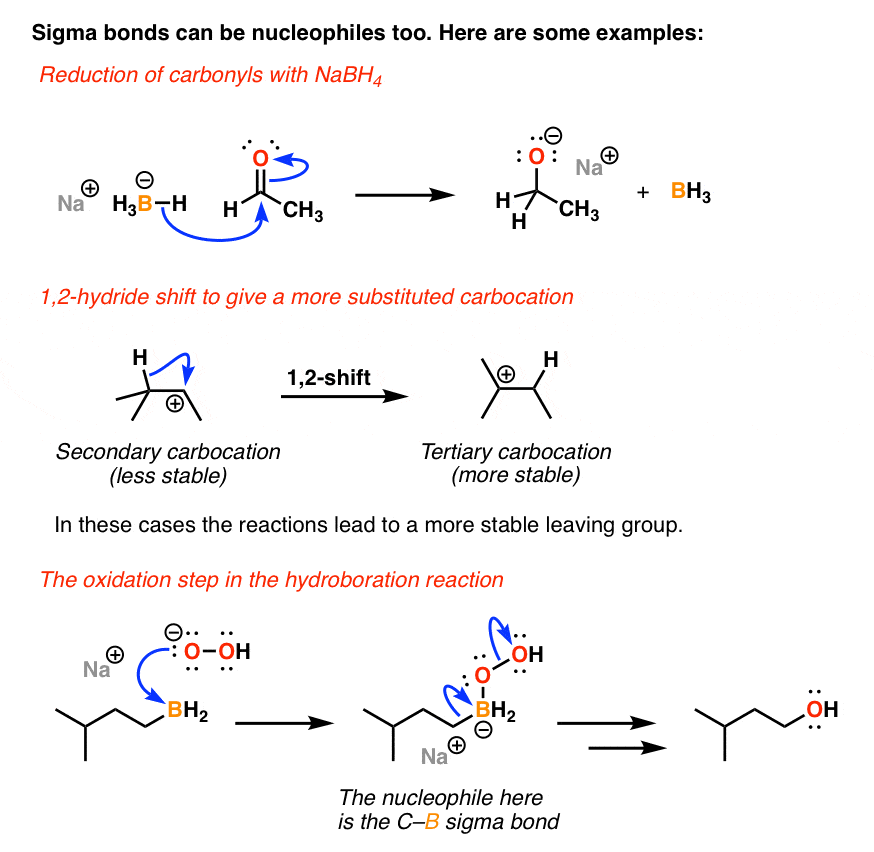
N1 reactions of allylic halides allylic halides and sulfonates are more reactive toward than simple alkyl halides toward nucleophilic substitution by the s n1 mechanism cc hc h h ch 3 ch 3 cc hc h h cl ch 3 ch 3 h h cc hc h h oh ch 3 ch 3 cc cch 3 ch 3 h oh h h cc cch 3 ch 3 h cl h h resonance stabilized carbocation intermediate over 100x. Due to the stability of the carbocation allyl compounds radially form intermediates during the reaction. The double bonded carbon atoms can be classified as vinylic and allylic carbon atoms. Allyl form a stable carbocation because of the electron delocalization.
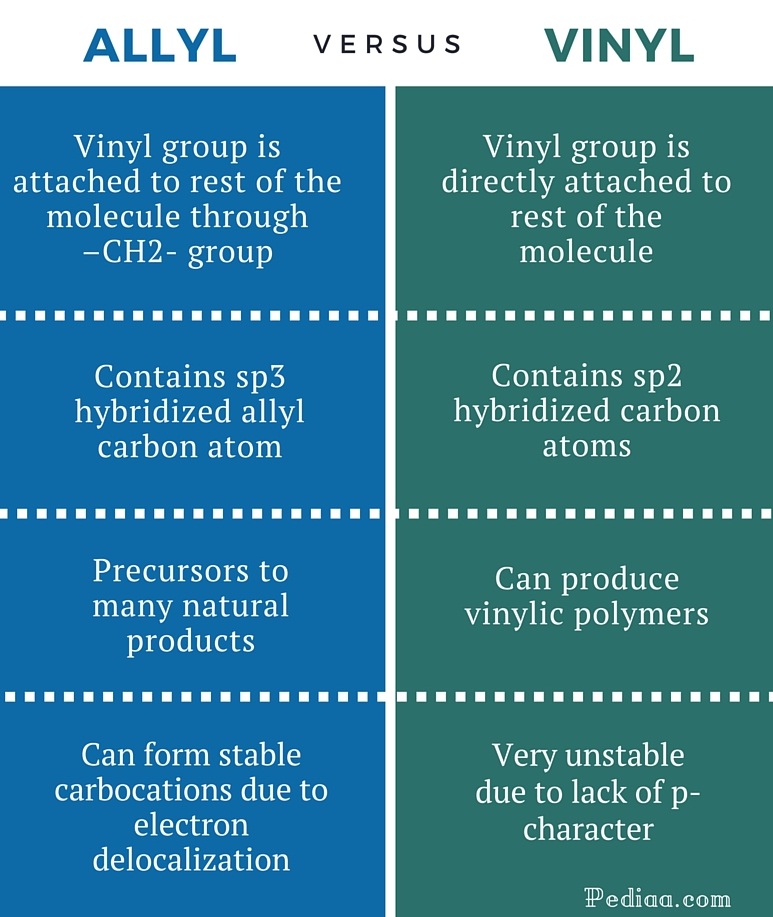
A vinyl carbocation has a positive charge on the same carbon as the double bond. Allyl group gets attached to any other group of atoms through ch 2 group. An allylic carbon is one that is directly attached to a pi bond. An allylic carbocation is a resonance stabilized carbocation in each of the two resonance forms of which the formal charge of 1 is on an allylic carbon.
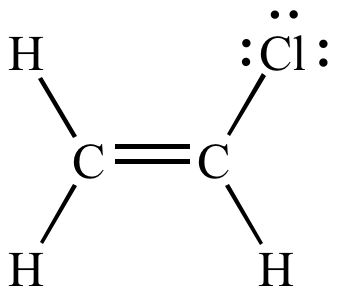
Both carbons involved in this bond are vinylic carbons. Vinylic carbocations are unstable as they lack p character. An allylic system has a minimum of 3 carbons. Do not confuse an allylic group with a vinyl group.
As expected from its sp hybridization the vinyl cation prefers a linear geometry. The overall charge on the carbocation remains unchanged but some of the charge is now carried by the alkyl groups attached to the central carbon atom. The allylic carbon and the nearby double bond. It is sp 2 hybridized.
Vinylic carbon makes a double bond with another carbon which is also sp 2 hybridized.